

Preparation and Cleaning
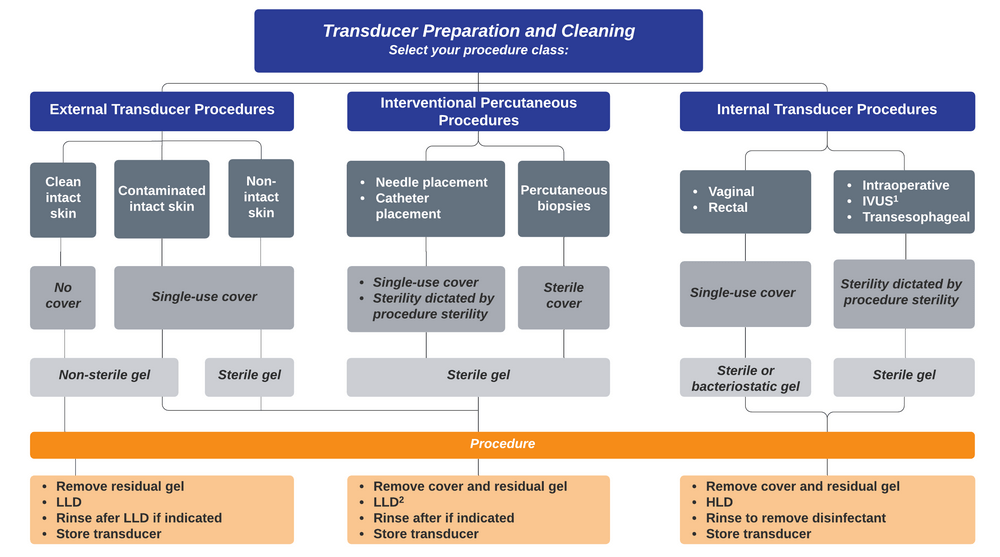
- Transducers used on clean, intact skin do not need a probe cover but do require low-level disinfection after each use.
- Transducers used during percutaneous procedures (vascular access, thoracentesis, paracentesis, arthrocentesis, regional anesthesia, etc) or those used on non-intact skin should be covered with a single-use sterile probe cover and gel should be sterile from a single use packet. The transducer should undergo low-level disinfection between uses.
- If the cover fails or if an external transducer comes into contact with blood or body fluids, it should undergo low level decontamination with a product active against bloodborne pathogens (hepatitis B, hepatitis C virus, and HIV) and TB. Caviwipes and Super SaniCloths do meet this criteria.
- Internal transducers with mucosal contact (eg, endocavitary transducers for intra-oral or transvaginal exams and transesophageal transducers) should be covered with a single-use probe cover and undergo high-level disinfection between uses. (Our hospital uses a Trophon device for HLD.)
Cleaning: the removal of visible soiling from the surfaces and lumens of equipment by a manual or mechanical process, commonly with water and detergent or an enzymatic cleaner.
Disinfection: the thermal or chemical destruction of pathogenic and other types of microorganisms. Disinfection is less lethal than sterilization as it destroys most recognized pathogenic microorgansims, but not necessarily all microbial forms (eg, bacterial spores).
High Level disinfectants: destroy almost all microorganisms including HPV and most bacterial spores.
Low Level Disinfectants: destroy most bacteria (this includes TB in some cases) some viruses and some fungi.
There are many branded products that provide low level disinfection. The two we have the best access to are Caviwipes and Super SaniCloth (this is by institutional decision – I don’t have a dog in the fight). Both cover TB. Both come in convenient single use cloths in self contained plastic tubs. In the past, our machines were compatible with Caviwipes but not Super SaniCloth. However, the GE Venue, our new machine, is approved for use with both. So, purple tops are now approved.
A similar situation arises in regards to probe covers. Although some sources say that using adhesive barriers (brand names might include Tegaderm or Opsite) are adequate as probe cover for some procedures, they are not approved for this use. There are issues with the pore size as well as sticking things to the ultrasound probe. Therefore, we use a true sleeve probe cover when any cover is indicated – even peripheral IV placement. (The probe cover is not expensive and comes with a packet of sterile gel, which you would have to have gotten anyway).
- Guideline for Ultrasound Transducer Cleaning and Disinfection. Ann Emerg Med. 2018 Oct;72(4):e45-e47. doi: 10.1016/j.annemergmed.2018.07.035. PMID: 30236343.
- Guidelines for Cleaning and Preparing External- and Internal-Use Ultrasound Transducers and Equipment Between Patients as well as Safe Handling and Use of Ultrasound Coupling Gel. AIUM. https://www.aium.org/officialStatements/57