Are these interchangeable? This came up recently. Pediatricians, family doctors, and everyone else that provides primary care might not know whether to laugh or cry at our ignorance, but this is not in our wheelhouse despite the fact that we boost a lot of tetanus immunity. Let’s look at it.
Are they the same?
No. Both are vaccines that induce immunity against diphtheria, tetanus, and pertussis; but they are not interchangeable.
What is in them?
The vaccines against tetanus and diphtheria are both inactive, manufactured versions of the toxins secreted by the causative agents of these diseases (Clostridium tetani and Corynebacterium diphtheria, respectively) (1). These inactive, manufactured versions of toxins are called toxoids. The pertussis vaccine, on the other hand, is not a true toxoid. It consists of acellular antigens, which are used to induce an immune response to the causative bacteria, Bordetella pertussis. (“Acellular” as opposed to whole bacterial cells as were used in the past.)
So, what’s the difference?
The two vaccines contain the same things; the differences are in the amounts. DTaP is used in children less than seven years old with the goal of inducing or building immunity. It has “full doses” of all three components. Tdap, on the other hand, is used in adolescents and adults. It has a “full dose” of tetanus toxoid, but “reduced doses” of the diphtheria toxoid and the acellular pertussis antigens.
These difference are represented in the names by capital and lower case letters.
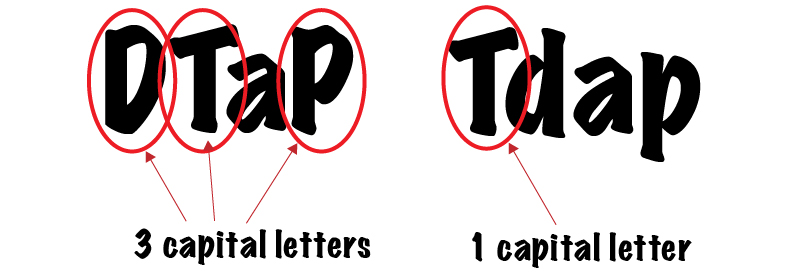
The three capital letters in DTaP represent the fact that it has full doses of all of three components. Tdap, on the other hand, only has a capital T, which represents the full doses of tetanus toxoid. It has lower case d and p, however, representing lower concentrations of the diphtheria toxoid and acellular pertussis antigens.
Interestingly, there are handful of manufacturers of the DTaP vaccine, and they don’t all contain the same amount of the toxoid/antigens. They do all, however, contain “at least” a standard amount of each (2).
Confusing details
The names
I can’t think of anything more confusing than the names DTaP and Tdap. The capital letter scheme is helpful when they are written, but in conversation they sound very similar to those unfamiliar with their differences.
Why age seven as a cutoff?
Higher doses of diphtheria toxoid and pertussis antigens are needed in children to stimulate the necessary immune response and are tolerated relatively well. Older children and adults, however, get an adequate response from the lower dose of those components. The goal seems to be to give the lowest dose that will generate the needed immune response so as to minimize side effects like discomfort and localized inflammatory reactions.
What would happen if we gave a child less than seven years old a Tdap instead of a DTaP?
I wouldn’t be harmful per se, but would be considered “invalid” in terms of their immunization schedule. They would still have to get the DTaP because the small d and small p are not enough to generate an adequate immune response. So, in terms of a tetanus vaccine, it would suffice. But, their primary care provider cannot check them off for that DTaP dose; so in that sense, it would have been for naught.
Is there anything to consider in older patients?
There are two brand names for the Tdap vaccine available in the US: Boostrix and Adacel. Boostrix is FDA approved for people over the age of 10 (including those 65 and older), while Adacel is only approved for ages 10 through 64. The lack of approval for Adacel in this age group, however, was not due to evidence of harm or lack of efficacy; rather it just wasn’t studied in that population. The CDC recommends Boostrix in patients 65 and older, but specifically states that if a patient needs the vaccine and you only have access to Adacel, “providers may administer the Tdap vaccine they have available, and it will be valid” (3).
Let’s wrap it up
DTaP and Tdap contain the same things, but at different concentrations. They are indicated for people of different ages. DTaP is for children less than seven, while Tdap is for everyone else. Giving a child less than seven years old a Tdap rather than a DTaP is not dangerous, but is inadequate in terms of their vaccine schedule. They will still have to get a DTaP in addition to that Tdap.
Elderly patients should receive a Tdap when clinically indicated, and although Boostrix is the only brand that is FDA approved in that population, the CDC acknowledges that off label use of Adacel is a valid option if that is all that is available in the moment.
References
- https://www.ncbi.nlm.nih.gov/books/NBK545173/
- https://www.cdc.gov/vaccines/vpd/dtap-tdap-td/hcp/about-vaccine.html
- https://www.cdc.gov/pertussis/hcp/vaccine-recommendations/index.html
