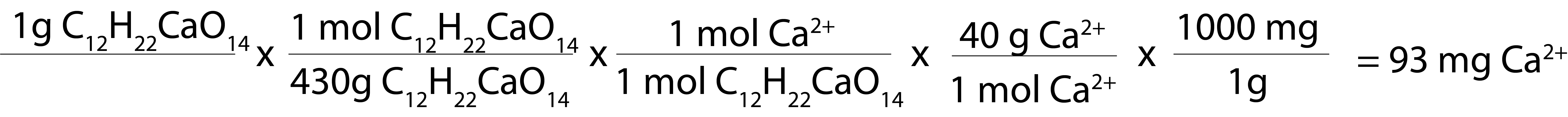When we need to give intravenous calcium for hyperkalemia, we generally have two options: calcium chloride and calcium gluconate. We think of calcium chloride as being “stronger,” but what exactly does that mean? Let’s look at.
Calcium chloride comes in prefilled syringes for emergent use and is in the crash cart. We get lazy about the dose because it is so easy to ask for “an amp of calcium.” In most cases, however, we don’t actually want to give calcium chloride. We need to use its little brother: calcium gluconate. But what is the dose of that? Calcium gluconate does not (at least in the EDs I’ve worked in) come in prefilled syringes. We have to actually know what we are doing to give it. And, why is one stronger or more dangerous than the other?
First, lets consider the issue of “stronger”
What is the difference?
Calcium is calcium. The difference between the two is the size of the rest of the molecule. As we see below, calcium chloride (which is actually calcium chloride dihydrate in the solutions we use) is a comparatively small molecule with a molar mass of 147 g/mol.

Calcium gluconate, on the other hand, is a much larger molecule. Its molar mass is 430 g/mol. (It actually is in a hydrate form as well, but these are the numbers used in all of the “classic” calculations.)

For cardiac arrest due to hyperkalemia, the recommended dose of calcium is one gram of calcium chloride. For hyperkalemia with concerning ecg changes but not cardiac arrest, most (but not all) sources recommend one gram of calcium gluconate (some recommend more). So, the dose of EITHER is (at least for the indication of hyperkalemia) one gram, and they both come as 10% solutions.
Remember that in terms of medications, “percent” has the units of “grams per 100 milliliters” or “grams per deciliter.” So, a 10% solution would be 10 grams in 100 milliliters, which is 1 gram in 10 milliliters.
- 1 g of calcium chloride at 10% is a 10 ml solution
- 1 g of calcium gluconate at 10% is also a 10 ml solution
However, due to the differences in their molar mass, 1 gram of calcium chloride contains three times as many calcium atoms (“elemental calcium”) as 1 gram of calcium gluconate.
If we pile up a gram of each and count the calcium atoms, the difference becomes easy to see.


- One g of calcium chloride has 273 mg of elemental calcium*.
- One g of calcium gluconate has 93 mg of elemental calcium*.
This is why we say calcium chloride contains three times as much calcium as calcium gluconate. On a gram by gram basis, it does. By comparison, 1 tablet of extra strength tums has 300 mg of elemental calcium (per uptodate).
So, to get the same amount of elemental calcium per gram of calcium chloride, we would need to give three grams of calcium gluconate. Clearly, we do not need that much to treat ecg changes because the recommended dose is only one gram of calcium gluconate. So, we need not think of the one gram of calcium chloride as the “minimum” or the “standard”. A third of its dose of elemental calcium usually does the job, and we can always give more if that QRS complex doesn’t narrow with the single gram of calcium gluconate.
*the math is shown below if anyone is interested
Now consider why one is riskier
Calcium chloride is very caustic to tissues when extravasated. We should only use it in the most emergent of situations and ideally only through a central line. Its osmolality is 2,040 mosm/L when used at the strengths described above. Ten percent calcium gluconate, on the other hand, has a reported osmolality of 680 mosm/L. Whether you know it or not, your pharmacy or nurses dilute that even further in D5W to achieve a more physiologic – and less caustic – osmolality.
What’s the bottom line?
If you only remember one thing, it should be “1 gram“. Exactly how much elemental calcium you are giving takes more (and probably unnecessary) thought, but in the moment, that will get you through.
- 1 g of calcium chloride for cardiac arrest due to hyperkalemia (CVL if possible)
- 1 g of calcium gluconate for concerning ecg changes due to hyperkalemia**

**you can always give more if needed.
(Maybe it goes without saying that for any other indications, you would use varying amounts of calcium gluconate if intravenous calcium had to be given.)

Both formulations come as 10% solutions, what does that mean?
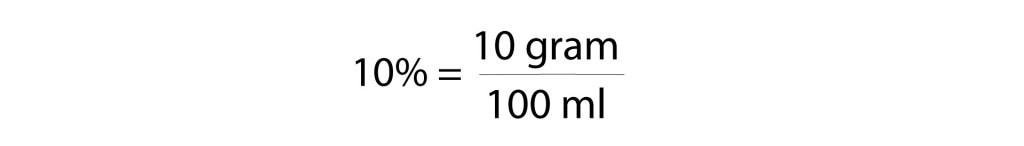
What is the volume of a 10% solution that has 1 gram of solute?
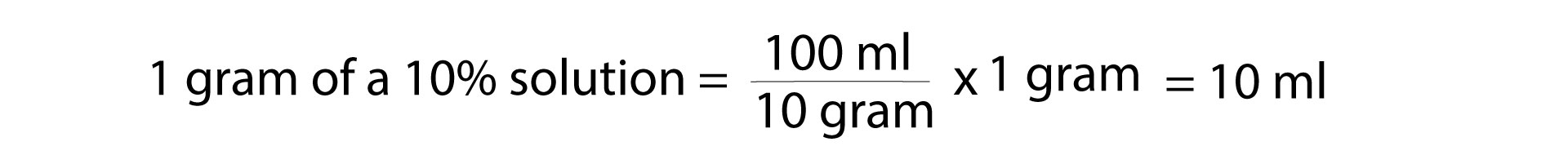
How much elemental calcium is in 1 gram of Calcium chloride?
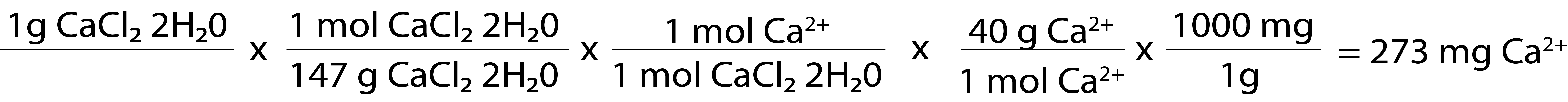
How much elemental calcium is in 1 gram of Calcium gluconate?